Equiptrack products and services are based only on information supplied to Equiptrack. Equiptrack does not have the complete history of every unique piece of equipment. Use the Equiptrack search as one important tool, along with an equipment inspection and functional check, to make a better decision about purchasing your next piece of used medical equipment.
Urgent Recall: ResMed AirFit N20 Nasal Mask
Urgent Recall: ResMed AirFit N20 Nasal Mask
Introduction:
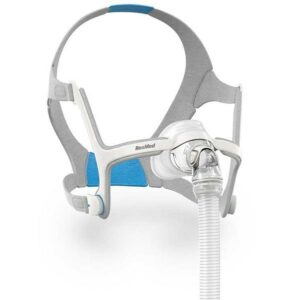
In a recent development, ResMed Ltd., a leading manufacturer of medical devices, has issued an urgent recall for its popular AirFit N20 Nasal Mask. The recall is related to potential magnetic interference with active medical implants, prompting the need for updated contraindications and warnings. The U.S. Food and Drug Administration (FDA) has determined that the root cause of the recall lies in the device’s design.
FDA Recall Link:
Does this apply to me?
– If you do not use a mask with magnets, the updated contraindications and warnings do not apply to you.
– The updated contraindications for ResMed masks with magnets only apply if you, or anyone in close physical contact with you while using the mask (e.g., bed partner), has a certain implant or medical device that is identified below.
– If you use a ResMed mask with magnets, you should also be aware of the updated warning noted below.
Introduction:
In a recent development, ResMed Ltd., a leading manufacturer of medical devices, has issued an urgent recall for its popular AirFit N20 Nasal Mask. The recall is related to potential magnetic interference with active medical implants, prompting the need for updated contraindications and warnings. The U.S. Food and Drug Administration (FDA) has determined that the root cause of the recall lies in the device’s design.
FDA Recall Link:
Does this apply to me?
– If you do not use a mask with magnets, the updated contraindications and warnings do not apply to you.
– The updated contraindications for ResMed masks with magnets only apply if you, or anyone in close physical contact with you while using the mask (e.g., bed partner), has a certain implant or medical device that is identified below.
– If you use a ResMed mask with magnets, you should also be aware of the updated warning noted below.

Reason for Recall:
The AirFit N20 Nasal Mask, known for its efficacy in providing a non-invasive interface for channeling airflow to patients, is under scrutiny due to magnets embedded in the mask. These magnets have the potential to interfere with active medical implants that interact with magnets, as well as metallic implants or objects containing ferromagnetic material. To address this issue, ResMed is updating contraindications and warnings regarding the safe distance to be maintained from medical devices and implants while using the mask.
FDA’s Determination:
The FDA has identified the design of the device as the cause of the recall. This determination emphasizes the importance of ensuring the safety and compatibility of medical devices, especially those involving magnetic components that may impact other implanted medical devices.
Recall Scope:
All mask lots used with User Guide 638218/version 2020-02, 638243/version 2020-06, and prior.
Recall Quantity:
The recall affects 7,724,968 AirFit N20 Nasal Masks.
Recall Actions:
ResMed has taken swift action to address the issue and ensure the safety of patients using the affected masks. On November 20, 2023, the company posted “Urgent Field Safety Notices” and FAQs on its website at www.resmed.com/magnetupdate. Healthcare providers and distributors were also notified through email.
The following actions were recommended:
1.Physician Notification: Prescribing physicians and healthcare professionals were advised to be informed about the updated labeling (contraindications and warnings).
2.Patient Notification: Patients currently using the affected mask were instructed to contact their mask provider for a replacement if they are now contraindicated. ResMed provided a patient letter explaining the updated labeling.
3.Replacement Masks: Contraindicated patients were to be provided with a replacement mask without magnets promptly. In cases where an alternative mask was not available, patients were instructed to consult with their physician.
4.Consulting Physicians: Patients were advised to consult their physician or the manufacturer of their implant or other medical device for additional information on potential adverse effects related to magnetic fields.
5.Alternative Mask Options: For alternative mask options, patients were encouraged to contact ResMed or Customer Service representatives.
6.Customer Acknowledgement: Healthcare providers and distributors were required to complete and return a customer acknowledgement form via email to magnetresponse@resmed.com.
Contact Information:
For any queries or concerns, individuals are urged to contact their local ResMed representative or visit www.resmed.com/contact.
Conclusion:
ResMed’s proactive approach to addressing the potential magnetic interference issue with the AirFit N20 Nasal Mask highlights the company’s commitment to patient safety. This recall serves as a reminder of the critical importance of thorough testing and evaluation in the design and manufacturing of medical devices to prevent unforeseen issues and ensure the well-being of patients. If you or someone you know is using the affected mask, it is crucial to follow the provided instructions and seek prompt replacement to avoid any potential risks associated with magnetic interference.
Additional Information:
Updated Contraindications:
– Masks with magnetic components are contraindicated for use by patients where they, or anyone in close physical contact while using the mask, have the following:
– Active medical implants that interact with magnets (i.e., pacemakers, implantable cardioverter defibrillators (ICD), neurostimulators, cerebrospinal fluid (CSF) shunts, insulin/infusion pumps)
– Metallic implants/objects containing ferromagnetic material (i.e., aneurysm clips/flow disruption devices, embolic coils, stents, valves, electrodes, implants to restore hearing or balance with implanted magnets, ocular implants, metallic splinters in the eye)
Updated Warning:
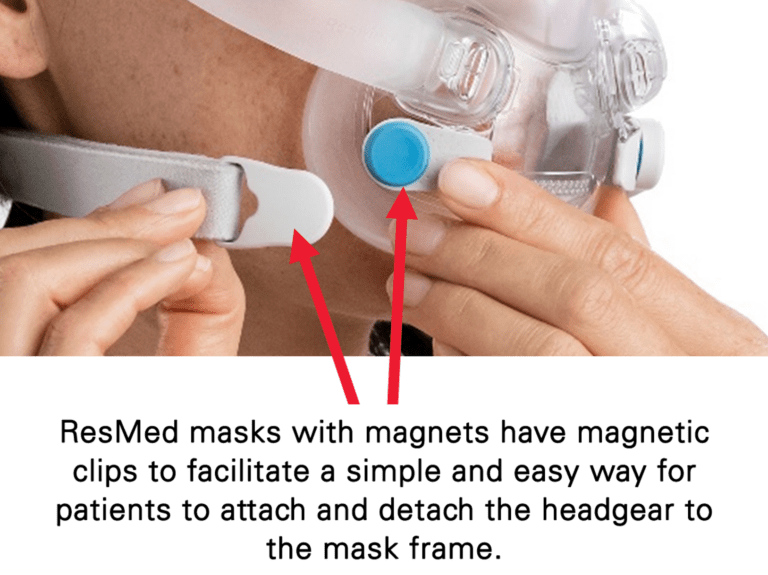
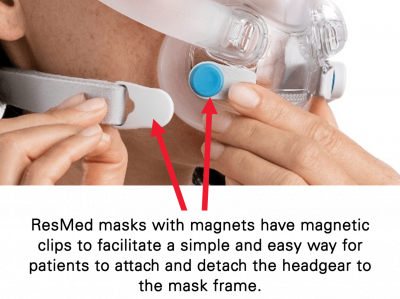
– Keep the mask magnets at a safe distance of at least 6 inches (150 mm) away from implants or medical devices that may be adversely affected by magnetic interference. This warning applies to you or anyone in close physical contact with your mask. The magnets are in the frame and lower headgear clips, with a magnetic field strength of up to 400mT. When worn, they connect to secure the mask but may inadvertently detach while asleep.
– Implants/medical devices, including those listed within contraindications, may be adversely affected if they change function under external magnetic fields or contain ferromagnetic materials that attract/repel to magnetic fields (some metallic implants, e.g., contact lenses with metal, dental implants, metallic cranial plates, screws, burr hole covers, and bone substitute devices). Consult your physician and manufacturer of your implant / other medical device for information on the potential adverse effects of magnetic fields.
– Note, not all models or variants of medical devices listed in the contraindications are affected by external magnetic fields. If you are not sure whether an implant or medical device falls under the contraindications, or you require additional information on the potential adverse effects of magnetic fields for your particular implant or medical device, please contact your physician/doctor.
Which ResMed masks contain magnets?
– Use the links below to find more information on each ResMed mask that features magnetic headgear clips. Product availability may differ in each country.
– https://www.resmed.com/en-us/cpap-mask-magnet-clip-guidelines/

