Equiptrack products and services are based only on information supplied to Equiptrack. Equiptrack does not have the complete history of every unique piece of equipment. Use the Equiptrack search as one important tool, along with an equipment inspection and functional check, to make a better decision about purchasing your next piece of used medical equipment.
Medical Device Recalls: Getinge Maquet SAS OR Light Systems
Medical Device Recalls: Getinge Maquet SAS OR Light Systems
Introduction:
Getinge, a leading manufacturer of OR Light Systems, has issued multiple Class 2 medical device recalls for the below Lighting Systems.
FDA Recall Links:
Z-0757-2024 – Maquet Blueline Series 30/80 OR Light Systems
Z-0758-2024 – Maquet Lucea – Lucea10/40, Lucea50/100 OR Light Systems
Z-0759-2024 – Maquet Prismalix OR Light Systems
Z-0760-2024 – Maquet Hanaulux HLX2000 OR Light Systems
Z-0761-2024 – Maquet Axcel / Axcel + OR Light Systems
Z-0762-2024 – Maquet Orchide OR Light Systems
Z-0763-2024 – Maquet XTen OR Light Systems
Z-0764-2024 – Maquet PowerLED/HLED and PowerLED300 OR Light Systems
Z-0765-2024 – Maquet G8 / G8E OR Light Systems
Z-0766-2024 – Maquet Hanaulux 2006/ 2007, Blue 100, Blue 130/ 90, Blue Series 30/ 80, and Prismatic OR Light Systems
Z-0767-2024 – Maquet Hanaulux HLX3000 OR Light Systems
Z-0768-2024 – Maquet PowerLEDII OR Light System
Z-0769-2024 – Maquet Rolite OR Light Systems
Z-0770-2024 – Maquet Volista, Volista Access, Volista Access II, Volista Standop OR Light System
Z-0771-2024 – Maquet Equipment OR Light Systems
Recall Scope:
All Models, All Catalog Numbers.

Introduction:
Getinge, a leading manufacturer of OR Light Systems, has issued multiple Class 2 medical device recalls for the below Lighting Systems.
FDA Recall Links:
Z-0757-2024 – Maquet Blueline Series 30/80 OR Light Systems
Z-0758-2024 – Maquet Lucea – Lucea10/40, Lucea50/100 OR Light Systems
Z-0759-2024 – Maquet Prismalix OR Light Systems
Z-0760-2024 – Maquet Hanaulux HLX2000 OR Light Systems
Z-0761-2024 – Maquet Axcel / Axcel + OR Light Systems
Z-0762-2024 – Maquet Orchide OR Light Systems
Z-0763-2024 – Maquet XTen OR Light Systems
Z-0764-2024 – Maquet PowerLED/HLED and PowerLED300 OR Light Systems
Z-0765-2024 – Maquet G8 / G8E OR Light Systems
Z-0766-2024 – Maquet Hanaulux 2006/ 2007, Blue 100, Blue 130/ 90, Blue Series 30/ 80, and Prismatic OR Light Systems
Z-0767-2024 – Maquet Hanaulux HLX3000 OR Light Systems
Z-0768-2024 – Maquet PowerLEDII OR Light System
Z-0769-2024 – Maquet Rolite OR Light Systems
Z-0770-2024 – Maquet Volista, Volista Access, Volista Access II, Volista Standop OR Light System
Z-0771-2024 – Maquet Equipment OR Light Systems
Recall Scope:
All Models, All Catalog Numbers.


Reason for Recall:
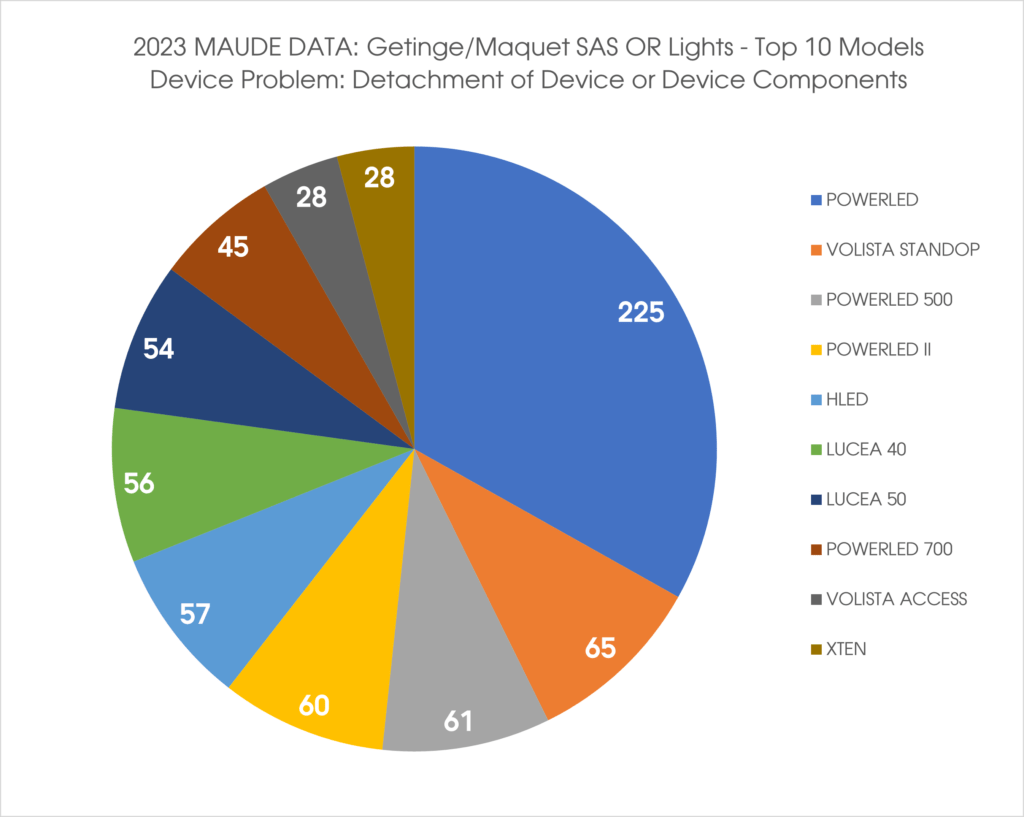
Getinge USA has stated that a potential for the light system to fall in the operating room is the reason for the recall.
Getinge has developed a comprehensive web site to provide additional information per model line per their Field Action Reference MSA-808092 at https://www.getinge.com/int/campaigns/maintenance-and-service-on-or-light-system/ .
FDA’s Determination:
The FDA has identified equipment maintenance as the cause of the recall.
Recall Quantity:
The recall affects 236,793 total units US Nationwide and Worldwide.
Recall Actions:
The following actions are listed in the Recall:
Light system fixing and other replacements:
Regarding:
– the fixing of the light systems: suspensions fixing screws, adapter fixing screws, bushing fixing screws
– the brake screws
– the safety segments
– the batteries, a dedicated instruction to illustrate and facilitate understanding of what to replace and what to order for replacement has been issued.
Contact Information:
Please complete and sign the MEDICAL DEVICE CORRECTION – RESPONSE FORM (page 7) to acknowledge that you have received this notification. Return the completed form to Maquet/Getinge by e-mailing a scanned copy to or-lights2023.qrc@getinge.com or by faxing the form to (866) 350-0897.

